Answer:
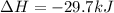
Step-by-step explanation:
Hello!
In this case, since the undergoing chemical reaction is:
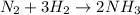
We first need to identify the limiting reactant given the masses of nitrogen and hydrogen:
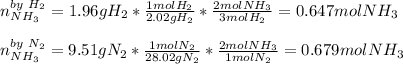
It means that only 0.647 moles of ammonia are yielded, so the resulting enthalpy change is:
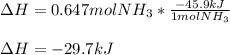
Best regards!