Answer : The electron-domain geometry around the 'A' atom is, octahedral.
Explanation :
As per given in the question, an
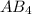 has two lone pairs of electrons on the atom 'A' and four number of monovalent atoms (B) are bonded to the central atom.
has two lone pairs of electrons on the atom 'A' and four number of monovalent atoms (B) are bonded to the central atom.
That means, the total number of electrons pairs = 2 + 4 = 6
The number of electrons pairs is 6 that means the hybridization will be
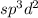 and the electronic or electron domain geometry of the molecule will be octahedral.
and the electronic or electron domain geometry of the molecule will be octahedral.
The electron domain geometry is also shown below.
Hence, the electron-domain geometry around the 'A' atom is, octahedral.