Alkanes are hydrocarbons with general formula
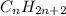 .
.
So alkane with two carbon atoms will be
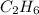 , alkane with four carbon atoms will be
, alkane with four carbon atoms will be
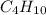 , alkane with six carbon atoms will be
, alkane with six carbon atoms will be
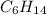 , alkane with eight carbon atoms will be
, alkane with eight carbon atoms will be
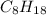 .
.
As the number of carbon atoms increase, the surface area will increase and thus the vanderwaal forces will also increase, and the boiling point will also increase.
Thus the approximate boiling point order is:
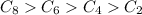
The approximate boiling points will be: 125° C >68° C > -1 °C > -89° C