Answer: In Na-23 atom having +1 charge, the number of
1) Protons = 11
2) Electrons = 10
3) Neutrons = 12
Explanation: There are 3 subatomic particles present in any nucleus: Protons, neutrons and electrons.
For a given
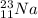
where, 11 = Atomic number
23 = Atomic mass
For an atom having +1 charge on it, the
Electronic configuration for
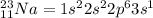
Electronic configuration for
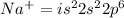 (It will loose one electron)
(It will loose one electron)
- Number of electrons = Sum of the superscripts in the above electronic configuration
Number of electrons = 10
- Number of protons = Atomic number
Number of protons = 11
- Atomic mass = Number of protons + Number of neutrons
Number of neutrons = 23 - 11 = 12