Answer: The correct option is A.
Explanation: Moles of Iron nail can be calculated by using the formula:
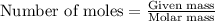 ...(1)
...(1)
Given mass of iron nail = 100 g
Molar mass of iron = 56 g/mol
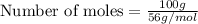
Number of moles of iron nail = 1.79 moles
The chemical equation for the formation of rust if given by:
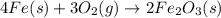
By Stoichiometry of the reaction,
4 moles of iron reacts with 3 moles of oxygen gas to produce 2 moles of rust.
So, 1.79 moles of iron will react with =
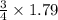 = 1.3425 moles of oxygen gas.
= 1.3425 moles of oxygen gas.
For oxygen gas,
Molar mass = 32 g/mol
Mass of oxygen can be calculated by using equation 1, we get
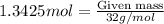
Mass of oxygen gas = 43 grams
Hence, the correct option is a.