Answer:
11.44
Step-by-step explanation:
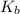 = Base ionization constant of ammonia =
= Base ionization constant of ammonia =
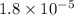
m = Mass of ammonia = 5 g
V = Volume of ammonia = 709 mL =
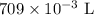
M = Molar mass of ammonia = 17.03 g/mol
Molaritiy of ammonia
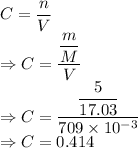
We have the relation
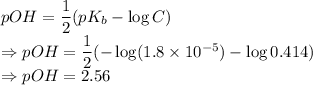
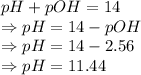
The pH of the solution is 11.44.