Answer:- Oxidation number of Cl does not change as it is -1 on both sides.
Explanations:- oxidation number of Mg on reactant side is 0 as it is in its elemental form(not combined with another element).
Oxidation number of hydrogen in its compounds is +1, so if H is +1 in HCl the oxidation number of Cl is -1 as the sum has to be zero.
On product side, Mg oxidation number is +2 as the oxidation number of alkaline earth metals in their compounds is +2.
Two Cl are present in magnesium chloride, so if Mg is +2 then Cl is -1.
Oxidation number of H on product side is 0 as it is present in its elemental for,
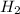 ,
,
So, it is only chlorine(Cl) whose oxidation number does not change for the given equation.