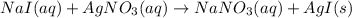Explanations:- If a reaction looks like,
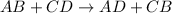 then it is called double displacement reaction as two ions are exchanged in this reaction.
then it is called double displacement reaction as two ions are exchanged in this reaction.
When sodium iodide and silver nitrate are added then a double displacement reaction takes place where iodide ion goes from sodium to solver and nitrate ion from silver to sodium. A precipitate of silver iodide forms in this reaction.