Answer:- 786 L of water is required.
Solution:- From given information, solubility of calcium carbonate is
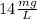 .
.
We are asked to calculate the volume of water required to dissolve 11 grams of calcium carbonate. Solubility is like the density of the solution and the mass is also given. So, this problem is like to calculate the volume when mass and density are given.
Density formula is:
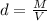
where d is density, M is mass and V is volume.
using this formula, we can calculate the volume but unit conversion is required as the mass is given in grams and the density gas milligrams. So, let's first convert 11 grams to milligrams. (1g = 1000mg)
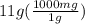
= 11000 mg
The formula could be rearranged for the volume as:
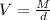
Let's plug in the values in it:
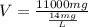
V = 785.71 L
It could be round to 786 L. So, 786 L of water is required to dissolve 11 grams of calcium carbonate. (Note- If significant figures are considered then it is round off to 790 L)