Answer:
248 mL of 5% w/v boric acid solution should be used to obtain the solution needed.
Step-by-step explanation:
We can calculate the volume of the 5% w/v boric acid solution needed to prepare the buffer solution 1.24% w/v using the following equation:
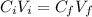
Where:
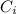 : is the concentration of the initial solution = 5% w/v
: is the concentration of the initial solution = 5% w/v
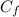 : is the concentration of the final solution = 1.24% w/v
: is the concentration of the final solution = 1.24% w/v
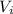 : is the volume of the initial solution =?
: is the volume of the initial solution =?
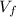 : is the volume of the final solution = 1 L
: is the volume of the final solution = 1 L
Hence, the volume of the 5% solution is:
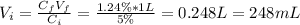
Therefore, 248 mL of 5% w/v boric acid solution should be used to obtain the solution needed.
I hope it helps you!