Answer:
Ease of use and readability.
Step-by-step explanation:
An atom of Oxygen, as an example, weighs 0.000...0001661 grams (with 23 zeros in front of "1661". In scientific notation this is written more concisely as
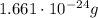
Still, in both cases the representation in units of grams is not easily readable and inconvenient to handle, especially when used in equations multiplying such mass with other measures. For this reason, the mass was agreed to be measured in atomic units, or a.u., which offer a numerically comparable baseline. An atomic unit for mass relates to the mass of an electron which is small enough to provide a good measurement unit. There are several variations to this so I recommend further reading if you need more detail.