Answer
12.3 years
Step by step Solution
Step 1
The first step is to use the radioactive decay formula to to calculate the radioactive constant for hydrogen 3. The radioactive formula is ,
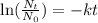 where
where
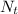 is the amount available at any time,
is the amount available at any time,
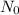 is the initial amount of substance,
is the initial amount of substance,
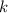 is the radioactive decay constant. We are given the values
is the radioactive decay constant. We are given the values
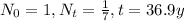 . We use these values to calculate the constant as follows,
. We use these values to calculate the constant as follows,
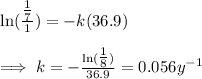
Step 2
The next step is to use the value for the decay constant to calculate the half life. We notice that at the half life, the value of the material remaining is 0.5. We show the calculation as shown below,
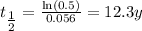
The half life is 12.3 years.