Answer: The correct answer is 1.
Explanation: There are various process in which a radioactive nuclei decays:
1) Alpha Decay: In this process, a heavier nuclei decays into lighter nuclei by releasing alpha particle. The mass number is reduced by 4 units.
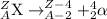
2) Electron capture: In this decay process, a parent nuclei absorbs an electron and gets converted into a neutron. Simply, a proton and an electron combines together to form a neutron. Mass number does not change in this process.
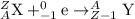
3) Gamma ray emission: It is a decay process in which an unstable nuclei gives excess energy by a spontaneous electromagnetic process. This decay releases
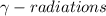 . This process does not change the mass number.
. This process does not change the mass number.
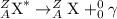
4) Positron emission: It is a type of decay process, in which a proton gets converted to neutron and an electron neutrino. This is also known as
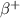 -decay. In this the mass number remains same.
-decay. In this the mass number remains same.
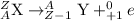
From the above information, we see that only alpha-decay is producing a change in the mass number of the nucleus.