The correct answer is A. N2O has 2 nitrogen atoms, whereas NO has only one.
For a chemical compound, the chemical formula depicts the proportions in which the atoms are present in that particular compound. So the chemical formulas indicate the type of elements and the number of atoms of each element present in the compound. The atoms of each element are represented by their chemical symbol and the number of times the element is present s represented as a subscript on that element.
In
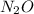 , the subscript on N is 2 so there would 2 N atoms and 1 O atom in each molecule of
, the subscript on N is 2 so there would 2 N atoms and 1 O atom in each molecule of
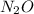 .
.
NO has one nitrogen atom and one Oxygen atom.