Answer:
We have
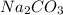 in an aqueous state
in an aqueous state
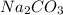 is a salt therefore being in an aqueous state will ionize
is a salt therefore being in an aqueous state will ionize
It will separate into the two ions that compose it.
When adding silver ions in an aqueous state a simple displacement reaction will occur

In a simple displacement reaction, an element reacts with a compound and takes the place of one of the elements of the compound, producing a different element and also a different compound.
General equation of a simple displacement reaction

In this case, silver displaces sodium because it is a more reactive metal.
After the reaction occurs, a precipitate of calcium carbonate is observed since this new compound is insoluble in water.