.0089mL
To find this, use the equation
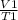 =
=
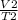
V1 being the volume and T1 being the temperature and T2 being the second temperature.
For all of the temperatures, since they're in celsius, you need to convert them to kelvin. To do this, add 273 to both numbers which will convert them to 281K and 303K respectively.
Hope this helps!