Answer:The approximate atomic mass of lead is 207.24 amu.
Step-by-step explanation:
Abundance of isotope (I) = 1.4% = 0.014
Atomic mass of the Isotope (I) = number protons + number of neutrons = 82 + 122 = 204 amu
Abundance of isotope (II) = 22.1 % = 0.221
Atomic mass of the Isotope (II) = number protons + number of neutrons = 82 + 125 = 207 amu
Abundance of isotope (III) = 24.1% = 0.241
Atomic mass of the Isotope (III) = number protons + number of neutrons = 82 + 124 = 206 amu
Abundance of isotope (IV) = 52.4% = 0.524
Atomic mass of the Isotope (IV) = number protons + number of neutrons = 82 + 126 = 208 amu
Average atomic mass of an element =
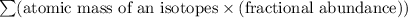
Average atomic mass of lead =
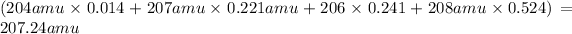
The approximate atomic mass of lead is 207.24 amu.