Answer: 207.24
Explanation: Atomic mass = no of protons + no of neutrons
1) 82 protons, 122 neutrons , mass number= 82+122 = 204
2) 82 protons, 125 neutrons, mass number= 82+125 = 207
3) 82 protons, 124 neutrons, mass number= 82+124 = 206
4) 82 protons, 126 neutrons, mass number= 82+126 = 208
Average atomic mass of an element =
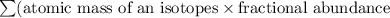
Average atomic mass of given element =
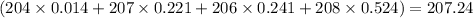
The approximate atomic mass of lead is 207.24.