Answer: The correct answer is Image 3.
Step-by-step explanation:
Lewis dot structure is defined as the structure which represents the number of valence electrons around the atom. The electrons are represented as dots.
From the structure, we can easily determine the number of bonding and non-bonding electrons. Non-bonding electrons are considered as lone pair of electrons.
Carbon is the 6th element of the periodic table having electronic configuration of
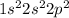
This element has 4 valence electrons.
Chlorine is the 17th element of the periodic table having electronic configuration of
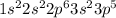
This element has 7 valence electrons.
The lewis dot structure for the compound
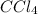 is given below in the image attached.
is given below in the image attached.
Hence, the correct answer is Image 3.