Answer:
(a)
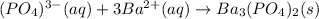
(b) 0.0136 moles.
Step-by-step explanation:
Hello!
(a) In this case, for the described chemical reaction and the requirement of the net ionic equation, we first need the complete molecular one:
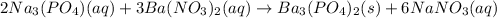
Now we split up the aqueous species into ions:
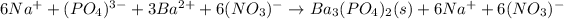
It means we can cancel out both sodium and nitrate ions are they are the spectator ones, in order to get the net ionic equation:
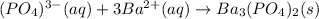
(b) Now, since 4.10 grams of barium phosphate precipitate is produced, we can compute the moles of sodium phosphate that reacted by using the molar mass of the barium phosphate and the 1:2 mole ratio between them:
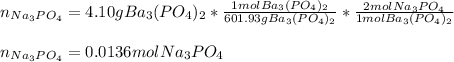
Moreover, the molarity of such solution was:
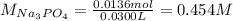
Best regards!