In a double displacement reaction, the cations and anions of two compounds exchange their places resulting in the formation of new compounds.
The given reactants are sodium phosphate (
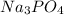 ) and copper (II) sulfate(
) and copper (II) sulfate(
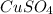 ). Sodium phosphate and copper sulfate react to form sodium sulfate and copper phosphate.
). Sodium phosphate and copper sulfate react to form sodium sulfate and copper phosphate.
The balanced chemical equation for the reaction between sodium phosphate and copper sulfate can be represented:
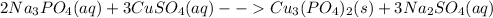
The product formed copper phosphate is insoluble solid and the other product sodium sulfate is soluble.