Answer: Option (B) is the correct answer.
Step-by-step explanation:
Ionization is defined as the production of ions in a solvent or solution. Whereas dissociation also means breaking of a chemical compound into ions or molecules when it is dissolved in water or polar solvent.
For example, when NaCl is dissolved in water then it will dissociate into sodium ions (
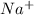 ) and chlorine (
) and chlorine (
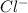 ) ions.
) ions.
Hence, we can conclude that ionization and dissociation processes produce solute ions in a solution.