Answer:
The reaction requires 243 grams of HF.
Step-by-step explanation:
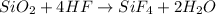
Moles of silicon dioxide =
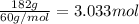
According to reaction 1 moles of silicon dioxide reacts with 4 moles of hydrogen fluoride.
Then 3.033 moles of silicon dioxide will react with:
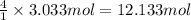 of hydrogen fluoride
of hydrogen fluoride
Mass of 12.133 moles of hydrogen fkluorode is:
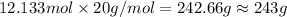
The reaction requires 243 grams of HF.