Solubility of a compound in water can be referred to as the amount of the compound that can be dissolved in 1 L of the solvent (water) at any given temperature. Solubility of a compound can be expressed in the units of g/L or mg/L.
Given that the solubility of calcium carbonate in water = 14 mg/L
We have to calculate the volume of water that can dissolve 11 g of calcium carbonate.
Converting 11 g calcium carbonate to mg:
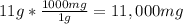
Volume of water that would dissolve 11000 mg calcium carbonate
=
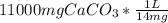
=785.7 L
Rounding the volume 785.7 L to two significant figures, we get 790 L water.
Therefore, we would need 790 L water to completely dissolve 11 g of calcium carbonate.