Answer: The two elements having same properties as calcium are magnesium and strontium.
Step-by-step explanation:
In a periodic table, elements are arranged in 18 vertical columns known as groups and 7 horizontal rows known as periods.
Elements arranged in a group show similar chemical properties because of the presence of same number of valence electrons.
Calcium is the 20th element present in Group 2 and Period 4 of the periodic table. The electronic configuration of this element is
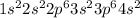
The number of valence electrons in Calcium are 2.
The elements having similar chemical properties as of calcium will be magnesium and strontium because they both have the same valence electrons that is 2.
Hence, the two elements having same properties as calcium are magnesium and strontium.