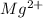Step-by-step explanation:
The species or elements which gain electrons and reduces itself are known as oxidizing agent or oxidant.
Ability of an element to act as an oxidizing agent depends on its electrode potential.
The electrode potential of
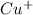 is 0.52 V.
is 0.52 V.
The electrode potential of
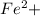 is -0.41 V.
is -0.41 V.
The electrode potential of
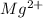 is -2.38 V.
is -2.38 V.
Greater is the value of electrode potential, stronger will be the oxidizing agent.
Therefore, rank of these species by their ability to act as an oxidizing agent are as follows.
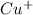 >
>
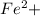 >
>