Answer: 51.45 grams of excess reagent is left after the completion of reaction.
Explanation: For the calculation of moles, we use the formula:
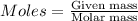 ....(1)
....(1)
Given mass = 92 grams
Molar mass = 28g/mol
Putting values in equation 1, we get:
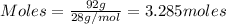
- For
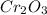
Given mass = 112 grams
Molar mass = 116g/mol
Putting values in equation 1, we get:
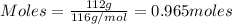
The reaction follows:
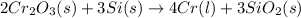
By Stoichiometry,
2 moles of
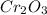 reacts with 3 moles of silicon
reacts with 3 moles of silicon
So, 0.965 moles of
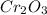 reacts with =
reacts with =
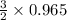 = 1.4475 moles of Silicon.
= 1.4475 moles of Silicon.
As, the moles of silicon is more than the required amount and is present in excess.
So, the excess reagent for the reaction is Silicon.
Moles of silicon remained after reaction = 3.285 - 1.4475 = 1.8375 moles
To calculate the amount of Silicon left in excess is calculated by using equation 1:
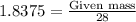
Amount of Silicon in excess will be 51.45 grams.