A reaction is apparently said to occur if it proceeds via the formation of a precipitate or a gaseous product, or a visible color change is observed after the reaction.
A)
 : This reaction occurs as there is a formation of white precipitate of barium sulfate.
: This reaction occurs as there is a formation of white precipitate of barium sulfate.
B) NaBr(aq)+KCl(aq)-->NaCl(aq)+KCl(aq): This reaction does not occur because all the ions just remain as spectator ions in the solution as the products are aqueous too.
C)
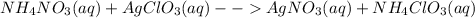 : This reaction does not occur because all the ions just remain as spectator ions in the solution as the products are aqueous too.
: This reaction does not occur because all the ions just remain as spectator ions in the solution as the products are aqueous too.
D)
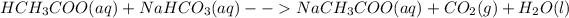 :This reaction can be observed as this proceeds via the formation of gas bubbles of carbon dioxide.
:This reaction can be observed as this proceeds via the formation of gas bubbles of carbon dioxide.
E)
 :This reaction occurs as there is a formation of white precipitate of barium hydroxide.
:This reaction occurs as there is a formation of white precipitate of barium hydroxide.
So the correct answers are:
A)

D)
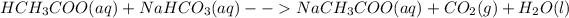
E)
