The given electronic configuration of the element is
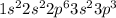
The element has 15 electrons as shown by the configuration. The element's valence shell is third shell and it has five electrons in the third shell (valence shell). Elements that fall in the same group of the periodic table have same number of valence electrons. Similarly, elements of the same period in the periodic table have same number of energy levels.
For the given element, there are three energy levels or shells. So it belongs to period 3. There are 5 valence electrons, so it belongs to the group 5A.
So the correct option is: d. period 3, group 5A