Given that the maximum acceptable concentration of fluoride in tap water = 1.5 mg/L.
1 mg/L is equivalent to 1 parts per million(ppm).
Converting 1.5 mg/L to ppm:
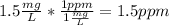
So the maximum acceptable concentration of fluoride in tap water in ppm is 1.5 ppm.
Finding out the volume of tap water that would contain 1.0 g fluoride:
Converting 1.0 g fluoride to mg:
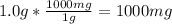
Taking the concentration of fluoride in tap water to be 1.5 mg/L,
Volume of tap water that contains 1000 mg fluoride
=
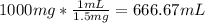
Rounding the volume to three significant figures, 667 mL of tap water.