Answer : The concentration of
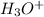 and
and
 are
are
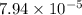 and
and
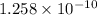 respectively.
respectively.
Solution : Given,
pH = 4.10
pH : pH is defined as the negative logarithm of hydronium ion concentration.
Formula used :
![pH=-log[H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/t1qqw59wbrmyd1orvr9wwtomxygflv3319.png)
First we have to calculate the hydronium ion concentration by using pH formula.
![4.10=-log[H_3O^+]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/4v4zkbrxirvjp2jlkhmxafsv0xuoqrga2j.png)
![[H_3O^+]=antilog(-4.10)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/btefd77rxmap7q3o9jkyzuk4plawgymu8r.png)
![[H_3O^+]=7.94* 10^(-5)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/2jjamgc733ph6382nbuggrrgjeiid4tzlp.png)
Now we have to calculate the pOH.
As we know,
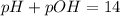
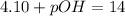
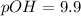
Now we have to calculate the hydroxide ion concentration.
![pOH=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bb6nd3dwmelw6hrf97fgv6xj1ptgdgg61f.png)
![9.9=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/p6zllipw9nc53fztbwnulczdg3524j11n1.png)
![[OH^-]=antilog(-9.9)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/5dthabwtaq98i4zci9ead13dkn1y1aht05.png)
![[OH^-]=1.258* 10^(-10)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/snpxjht7wklzhqe495bgc7phx1y93rd029.png)
Therefore, the concentration of
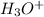 and
and
 are
are
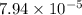 and
and
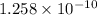 respectively.
respectively.