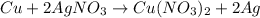Explanation:
Single displacement reactions is defined as the chemical reaction in which more reactive metal displaces less reactive metal in a chemical reaction. These type of reactions are studied with the help of reactivity series.
The metals which lie above in the series will displace the metals which lie below in the reactivity series.
General form of this type of reaction follows:
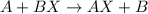
where, A is more reactive metal than B.
For the given reactants, the following reaction follows:
1) Zinc and Copper sulfate
The reaction will occur because zinc is more reactive than copper and will easily displace copper in a chemical reaction.
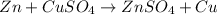
2) Aluminium and Copper sulfate
The reaction will occur because aluminium is more reactive than copper and will easily displace copper in a chemical reaction.
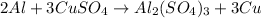
3) Zinc and Silver Nitrate
The reaction will occur because Zinc is more reactive than Silver and will easily displace silver in a chemical reaction.
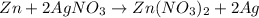
4) Copper and Silver Nitrate
The reaction will occur because Copper is more reactive than Silver and will easily displace silver in a chemical reaction.