Answer:
Empirical and molecular formulas are the same, C₅H₁₀O₂.
Step-by-step explanation:
Hello!
In this case, when determining the empirical and molecular formulas of organic compounds via combustion analysis, we first need to compute the moles of carbon and hydrogen via the yielded mass of carbon dioxide and water:
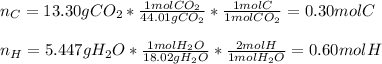
Next, we need to compute the mass of oxygen by subtracting the mass of carbon and hydrogen to the mass of the sample of the compound:
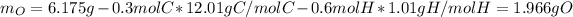
And consequently the moles:
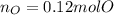
Now, we need to divide the moles of each atom by the fewest moles, it in this case, those of oxygen to obtain the subscripts in the empirical formula:
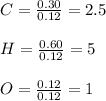
Thus, the empirical formula, taken the nearest whole number is:
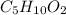
Now, if we divide the molar mass of the molecular formula (102.1 g/mol) by that of the empirical formula (102.1 g/mol) we infer they are both the same.
Best regards!