Answer: The correct option is 2.
Explanation: There are two types of nuclear reactions:
1) Nuclear fission: These reactions are defined as the reactions in which a heavier unstable nuclei breaks into two or more smaller stable nuclei.
2) Nuclear fusion: These reactions are the ones where two smaller nuclei fuse together or combine together to form a larger nuclei.
In the question, we need to find the fusion reaction which forms elements heavier than helium.
Option 1: In this fusion reaction occurs but the nuclei is Helium itself.
Option 2: In this also fusion reaction occurs and the nuclei is heavier than Helium which is Neon.
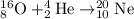
Option 3 and Option 4: These two reactions are nuclear fission reactions of Uranium-235 because one heavier element is breaking into more than 2 products.
Hence, the correct option is 2.