Answer: Option (a) is the correct answer.
Step-by-step explanation:
In an ionic bond is defined as the bond formed due to transfer of electron(s) from one atom to another.
An ionic bond will always be formed between a metal and a non-metal.
This is because an atom which loses its valence electrons (metals) acquires a positive charge and another atom which gains the electrons (non-metals) acquires a negative charge.
Hence, these opposite charges strongly gets attracted towards each other forming a strong bond.
Whereas in a covalent bond, there will be sharing of electrons between the combining atoms.
A covalent bond will always be formed between non-metals when they are present in neutral form.
For example,
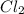 molecules is formed due to sharing of electrons between the two Cl atoms.
molecules is formed due to sharing of electrons between the two Cl atoms.
Thus, we can conclude that difference between an ionic bond and a covalent bond is that an ionic bond is a bond between charged atoms, while a covalent bond is a bond between neutral atoms.