Step-by-step explanation:
Thermochemical equation : it is a chemical reaction equation in which states of all reactants and products are written along with the energy change or enthalpy change (
 ) in a chemical reaction.
) in a chemical reaction.
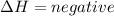 energy is released.
energy is released.
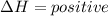 energy is absorbed.
energy is absorbed.
The Thermochemical equation for the given reaction will be :
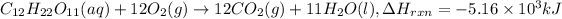
One mole of sucrose is oxidized by the 12 moles of oxygen to give 12 moles of carbon-dioxide, 11 moles of water and heat energy. the value of theis energy is equal the
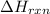 that is
that is
 .
.