Answer : The laboratory technician need to measure before making the solution is the mass of the solute.
Explanation :
Given : Molarity of NaOH = 2 M = 2 mole/L
Volume of solution = 500 ml
Molar mass of NaOH = 40 g/mole
For making the solution, we need the mass of NaOH.
Molarity : It is defined as the number of moles of solute present in on liter of solution.
Formula used :
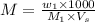
where,
M = molarity of the NaOH
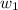 = mass of NaOH
= mass of NaOH
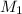 = molar mass of NaOH
= molar mass of NaOH
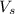 = volume of solution
= volume of solution
Now put all the given values in this formula, we get the mass of NaOH.
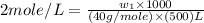
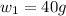
Therefore, the mass of NaOH is 40 gram.
As we know the molarity of solute, volume of solution and molar mass of the solute. Hence, the laboratory technician will need to measure only mass of the solute before making the solution.