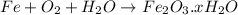Answer: B. They are both reactions of a substance with oxygen
Explanation: Burning is oxidation of a substance in the presence of oxygen. Example: Burning of natural gas which mainly consists of methane is an example of a chemical reaction in methane is burnt in the presence of oxygen and is transformed into carbon dioxide, water vapor and ash.
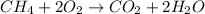
Rusting is the oxidation of iron in the presence of air and water to lead to formation of hydrated ferric oxide called as rust.