Answer : Carbon tetrachloride,
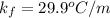 will show the greatest freezing point lowering.
will show the greatest freezing point lowering.
Explanation :
For non-electrolyte solution, the formula used for lowering in freezing point is,
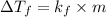
where,
 = lowering in freezing point
= lowering in freezing point
 = molal depression constant
= molal depression constant
m = molality
As per question, the molality is same for all the non-electrolyte solution. So, the lowering in freezing point is depend on the
 only.
only.
That means the higher the value of
 , the higher will be the freezing point lowering.
, the higher will be the freezing point lowering.
From the given non-electrolyte solutions, the value of
 of carbon tetrachloride is higher than the other solutions.
of carbon tetrachloride is higher than the other solutions.
Therefore, Carbon tetrachloride,
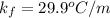 will show the greatest freezing point lowering.
will show the greatest freezing point lowering.