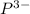Answer : The charge carried by a phosphide ion is, (-3)
Explanation :
In phosphide ion, the (-ide) indicates that the phosphide ion is the anion of phosphorous. So, we will have to add electrons.
As we know that the phosphorous belongs to group 5 and have 5 valence electrons.
The atomic number of phosphorous = 15
The electronic configuration of phosphorous is,
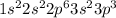
In phosphorous, the 'p' sub-shell is half filled. For fully filled 'p' sub-shell, we will need '3' electrons. By adding 3 electrons in 'p' sub-shell of phosphorous, (-3) charge will be created on phosphorous and named as phosphide ion.
Phosphide ion is written as,