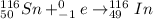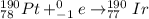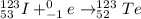Answer :
Electron capture : It is defined as the inner orbital electrons is captured by the nucleus converting a proton into a neutron.
Generally the electron capture equation is represented as,
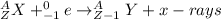
where,
A = atomic mass number
Z = atomic number
The electron capture equations are :