Answer:
9.98 g.
Step-by-step explanation:
Hello!
In this case, given the information found on the problem, we can see that the taken volume of water was 10 mL, and considering the formula of density:
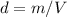
The mass would be computed as follows:
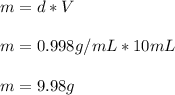
Thus, the nearest thousandth of a gram would also be 9.98 g.
Best regards!