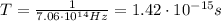1) Frequency:
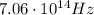
The frequency of electromagnetic radiation is given by:
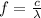
where
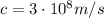 is the speed of light
is the speed of light
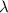 is the wavelength
is the wavelength
In this case, the wavelength of the radiation is
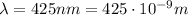
Therefore the frequency is
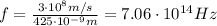
2) Period:
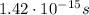
The period is equal to the reciprocal of the frequency of the wave:
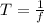
Using the frequency we found previously,
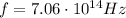 , we find:
, we find: