Answer : The combustion equation violates the law of conservation of matter is, (B)

Explanation :
According to the law of conservation of matter, the number of atoms of each elements of reactant side must be equal to the product side in the chemical reaction. Or we can say that the mass of reactant side must be equal to the mass of product side in the chemical reaction.
(A)
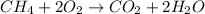
This combustion equation follow the law of conservation of matter.
(B)

This combustion equation does not follow the law of conservation of matter because in this reaction, the atoms of carbon and hydrogen elements are equal on both the sides but the atoms of oxygen element on reactant side are not equal to the product side.
(C)
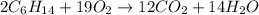
This combustion equation follow the law of conservation of matter.
(D)

This combustion equation follow the law of conservation of matter.
Hence, the combustion equation violates the law of conservation of matter is, equation (B)