Answer:
4, 7, 4, 6
Step-by-step explanation:
The law of conservation of mass states that in every chemical reaction, the mass of the reactant must be equal to the mass of the products: this means that the number of atoms of each element must be equal in the reactants and in the products.
If we choose the coefficients in this way:
4, 7, 4, 6
We see that the reaction becomes:
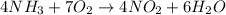
And we can verify that the number of atoms for each element in the reactants is equal to the product:
- Nitrogen (N): 4 atoms on the left, and 4 on the right
- Oxygen (O): 7 x 2 = 14 atoms on the left, 4 x 2 + 6 = 14 atoms on the right
- Hydrogen (H): 4 x 3 = 12 atoms on the left, 6 x 2 = 12 atoms on the right