Answer:
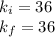
Step-by-step explanation:
Boyle's law states that the pressure of a given mass of an ideal gas is inversely proportional to its volume at a constant temperature.
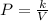
Where,
P = Pressure
V = Volume of the gas
k = Boyle's constant
Finding the initial Boyle's constant
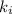 :
:
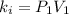
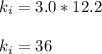
Finding the final value
 :
:
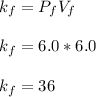
To verify Boyle's law, the initial Boyle's constant should be equal to the final Boyle's constant:
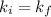
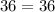
Therefore, the gas does obey Boyle's Law.