Answer: The expression for equilibrium constant is
![([NH_3]^2)/([H_2]^3[N_2])](https://img.qammunity.org/2019/formulas/chemistry/high-school/bdt172dubkdtf6fo0cp2qax2rutj1ky82q.png)
Explanation: Equilibrium constant is the expression which relates the concentration of products and reactants preset at equilibrium at constant temperature. It is represented as
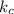
For a general reaction:
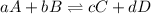
The equilibrium constant is written as:
![k_c=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2019/formulas/chemistry/high-school/bfhsw330mtmmxtwtmp4c9m8xdkgulix7w9.png)
Chemical reaction for the formation of ammonia is:
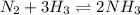
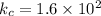
Expression for
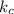 is:
is:
![k_c=([NH_3]^2)/([H_2]^3[N_2])](https://img.qammunity.org/2019/formulas/chemistry/high-school/qb9cdoivdjny4x1i5e79f9ig9alofyvycw.png)
![1.6* 10^2=([NH_3]^2)/([H_2]^3[N_2])](https://img.qammunity.org/2019/formulas/chemistry/high-school/6kr7o4aaiux3dsh0ilfkv96htwmt7wczlg.png)