According to Boyle's Law, The Volume of an Ideal gas is Inversely proportional to its Absolute Pressure at a Constant Temperature.
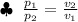
Assuming the Helium gas as an Ideal Gas and Temperature of the Atmosphere is Constant :
Here : p₁ = 274.2 kPa and p₂ = 52.2 kPa and v₁ = 2.5
⇒
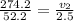
⇒
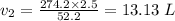
So, The New Volume is 13.13 Liter