Answer :
1) The correct option is, (A) Breaking a chemical bond releases energy.
2) The correct option is, (D) 43.2 Kcal are consumed when 2.00 moles of NO is produced.
Explanation for 1 :
Statement A is correct, because when the chemical bonds are breaking then the some amount of energy releases.
Statement B is incorrect, because when the energy is absorbed then the reaction is said to be endothermic and
 will be positive not negative.
will be positive not negative.
Statement C is incorrect, because the bond dissociation energy are always negative number not positive number.
Statement D is incorrect, because the stronger the bond, the more its bond dissociation energy not lower.
Explanation for 2 :
The given reaction is,
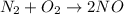
The value of
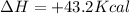
When the value of
 is positive then the energy is consumed.
is positive then the energy is consumed.
From the given reaction we conclude that
43.2 Kcal are consumed when 1 mole of
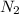 is reacted.
is reacted.
43.2 Kcal are consumed when 1 mole of
 is reacted.
is reacted.
43.2 Kcal are consumed when 2.00 moles of NO is produced.
Therefore, the correct option is, 43.2 Kcal are consumed when 2.00 moles of NO is produced.