Final answer:
To find the change in entropy when a monatomic ideal gas is given extra thermal energy, you need the initial temperature, which can be found using the initial thermal energy. Then, the entropy change is the heat added divided by this temperature. Without the initial temperature, the entropy change cannot be accurately determined.
Step-by-step explanation:
To calculate the change in entropy when the thermal energy of 1.00 mol of an ideal monatomic gas is increased by 24.0 J, we can use the following relationship for an ideal gas:
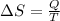
where
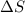 is the change in entropy, Q is the heat added to the system, and T is the absolute temperature of the system.
is the change in entropy, Q is the heat added to the system, and T is the absolute temperature of the system.
However, to proceed with this calculation, we need to know the initial temperature of the gas, which isn't provided in the question. Typically, one would use the initial thermal energy to find the initial temperature using the equation for the internal energy of a monatomic ideal gas:
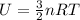
Where U is the internal energy, \(n\) is the number of moles, R is the ideal gas constant (8.314 J/mol·K), and T is the temperature in Kelvin.
Once the temperature is known, the change in entropy can then be calculated by dividing the amount of heat added (24.0 J) by the temperature.